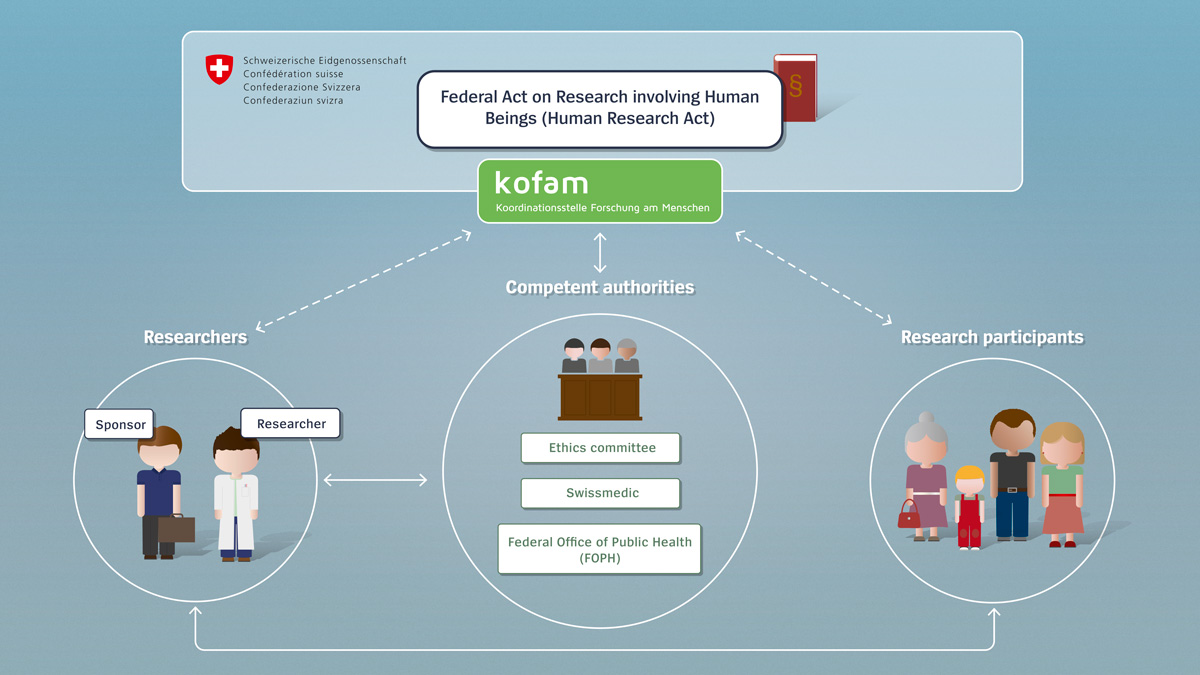
Researchers
The term “researcher” refers to the investigator – i.e. the person conducting the study – as well as the sponsor, that is to say the initiator of the project. In investigator-initiated studies, the investigator is also the sponsor. Every research project that falls within the scope of the Act must be authorised before it can be conducted – the researchers are thus obligated to submit an application for their research project with the authorities.
Furthermore, researchers are obliged to duly inform research participants in advance about the research project and obtain their voluntary consent to participate. During the project, they are also obliged to report any unusual incidents to the authorisation authorities.
Research participants
The term “research participants” refers to persons who are taking part in a project. They participate in a project of their own free will and after they have been duly informed in advance by the researchers. They have the right to withdraw from the project at any time. As long as they are taking part in the research project, however, they are obliged to adhere to the guidelines.
Authorisation authorities
The term “authorisation authorities” refers, on the one hand, to the cantonal ethics committees and, on the other hand, to the Swiss Agency for Therapeutic Products (Swissmedic) and, in some cases, to the Federal Office of Public Health (FOPH) and the Federal Office for the Environment (FOEN). The authorities review the applications and authorise the research projects. During the project, they are regularly informed by the researchers about unforeseen events, and they have the opportunity to intervene if necessary and, in extreme cases, discontinue a research project.
Federal Government
As a fourth stakeholder, the Federal Government dictates the framework conditions with the Human Research Act, also undertaking a coordinating role by way of the Coordination Office for Human Research (kofam) and providing information. Researchers can, for example, find out about authorisation procedures, and individuals can search a clinical trial register for research projects.
Further information
Cantonal ethics committees
The cantonal ethics committees are tasked with reviewing the ethical, scientific and legal aspects of a research project. In the case of clinical trials, they verify compliance with Good Clinical Practice (GCP). The ethics committees, furthermore, receive notifications and reports. Where necessary, they can impose additional conditions or suspend the authorisation for a research project.
- Ethics Committee of Berne
(Canton Berne)
- Ethics Committee of Geneva
(Canton Geneva)
- Ethics Committee for North-West and Central Switzerland
(Cantons Aargau, Basel-Landschaft, Basel-Stadt, Jura, Lucerne, Nidwalden, Obwalden, Solothurn, Schwyz, Uri and Zug)
- Ethics Committee of East Switzerland
(Cantons St. Gallen, Appenzell Innerhoden, Appenzell Ausserrhoden, Thurgau)
- Ethics Committee of Ticino
(Canton Ticino)
- Ethics Committee of Vaud
(Cantons Vaud, Neuchâtel, Fribourg, Valais)
- Ethics Committee of Zurich
(Cantons Zurich, Glarus, Graubünden and Schaffhausen; Principality of Liechtenstein)
Swissmedic
Swissmedic is the Swiss regulatory and supervisory authority for medicinal products and medical devices (therapeutic products). In the case of clinical trials of medical devices, Swissmedic ensures that product risks are taken into account and that product information reflects the current state of scientific knowledge and is correctly reflected in the protocol. In the case of clinical trials of medicinal products, Swissmedic reviews the safety and quality of the medicinal product. Swissmedic also authorises clinical trials of gene therapy and of transplant products. Swissmedic is authorised to inspect all clinical trials of therapeutic products or transplant products. In this manner the supervisory authority ensures compliance with statutory requirements and international guidelines.
Federal Office of Public Health
The Radiological Protection Division of the Federal Office of Public Health (FOPH) is responsible for the enforcement of radiological protection legislation. It is tasked with protecting people and the environment from the dangers of ionising radiation. If a research project includes investigations with unsealed or sealed radioactive sources (e.g. with a radiopharmaceutical) whose effective dose is above 5 mSv per annum, the FOPH will provide an opinion regarding the compliance with radiological protection legislation and dose estimation. For some clinical trials of therapeutic products capable of emitting ionising radiation, the FOPH also reviews compliance with radiological protection legislation and dose estimation.
Clinical trials of the transplantation of human organs, tissues and cells require authorisation from the FOPH’s Transplantation and Reproductive Medicine Section prior to execution. In the case of clinical trials of transplantation, the FOPH may carry out inspections at any time.
Furthermore, the FOPH’s Biosafety Section provides an opinion regarding clinical trials of gene therapy as well as clinical trials of genetically modified or pathogenic organisms.
Federal Office for the Environment
In case of clinical trials of gene therapy and clinical trials of genetically modified or pathogenic organisms, the Biotechnology Section of the Federal Office for the Environment (FOEN) assesses the environmental data and provides an opinion on the application. The Swiss Expert Committee for Biosafety (SECB) also provides an opinion.